Profit from stem cells - a great leap forward for medicine
From better drug testing to growing whole new organs – stem cell research is leading to some big investment opportunities. Matthew Partridge tips the best stocks to profit.

Get the latest financial news, insights and expert analysis from our award-winning MoneyWeek team, to help you understand what really matters when it comes to your finances.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Twice daily
MoneyWeek
Get the latest financial news, insights and expert analysis from our award-winning MoneyWeek team, to help you understand what really matters when it comes to your finances.

Four times a week
Look After My Bills
Sign up to our free money-saving newsletter, filled with the latest news and expert advice to help you find the best tips and deals for managing your bills. Start saving today!
From better drug testing to growing whole new organs stem cell research is leading to some big investment opportunities, writes Matthew Partridge.
Stem cells, which have the potential to turn into any other type of cell in the body, have fuelled some of the most heated debates in medical research in recent years. Ethical concerns over the use of stem cells from embryos were at the centre of a huge political debate in the US a few years ago.
Supporters of the research, including actors Michael J Fox (who suffers from Parkinson's disease) and the late Christopher Reeve (who was paralysed in an accident), argued that their potential for curing diseases was too great to ignore, and in the end they won the debate. Only Austria, Germany and Ireland now ban both research into and the production of such stem cell lines.
MoneyWeek
Subscribe to MoneyWeek today and get your first six magazine issues absolutely FREE

Sign up to Money Morning
Don't miss the latest investment and personal finances news, market analysis, plus money-saving tips with our free twice-daily newsletter
Don't miss the latest investment and personal finances news, market analysis, plus money-saving tips with our free twice-daily newsletter
Yet, despite hundreds of millions of dollars in funding from governments worldwide, there are currently few effective treatments derived from embryonic stem cells. There are also serious safety concerns. The cells are often rejected by the host body.
Worse still, when combined with drugs designed to prevent rejection, some studies show that they are associated with the development of cysts and tumours.
For example, a 2002 study of a Parkinson's treatment found that one in five rats injected with embryonic stem cells died of cancer. Some funding organisations, including one in California originally set up to explore stem-cell research, are now shifting funding away from the area.
Promising developments
The good news is that stem-cell research hasn't hit a brick wall. Far from it. While embryonic stem cells have disappointed, stem cells taken from adults have shown far more promise. In fact, they are now used to treat more than 70 different conditions.
What's more, a host of new treatments is in the pipeline. These fall into three categories: helping the body to repair itself; growing replacement organs for transplants; and improving the efficiency and safety of medical research.
Ever since the mid-19th century we've known that the body is made up of cells, some of which play a vital role in creating other cells. But it was not until 1961 that James Till and Ernest McCulloch formally proved the existence of adult stem cells (or somatic' stem cells). These cells divide and multiply, helping to renew other cells and enabling our bodies to repair everyday wear and tear.
But even before stem cells were officially discovered, doctors were using them. For example, the stem cells found in our bone marrow play a key role in the production of blood cells. Several diseases can damage the marrow's ability to produce blood, devastating the immune system.
But scientists found that if the faulty marrow is destroyed using drugs, then replaced with stem cells from a healthy person, then healthy bone marrow is produced in its place.
By the late 1960s, bone-marrow transplants had become standard treatment for several conditions, notably leukaemia. Today around 50,000 such transplants are performed each year.
Developments in the last decade suggest that these cells could have a far wider application than once thought. In 2003, for instance, a man from Michigan who had damaged his heart after accidentally shooting himself with a nail gun experienced a big improvement in heart function when bone-marrow cells were pumped into the damaged tissue via a catheter. A Brazilian study confirmed the results a year later. As a result, several companies have developed bone-marrow-based treatments for heart disease.
Bone-marrow therapy could also be used to treat the degenerative disease multiple sclerosis (MS). Last summer, doctors at Ottawa General Hospital published the results of a long-term clinical trial showing that the procedure could stop the progress of a particularly virulent form of MS. Brain scans taken two years after the treatment showed no new nervous system lesions, suggesting the disease had been halted in its tracks.
The biggest problem with bone-marrow transplants is finding an exact donor match to avoid the body rejecting the transplant. The odds of two unrelated people being a match are around 1 in 50,000.
Even with the advent of international donor banks, between one in ten and one in three people (depending on ethnicity) will be unable to receive treatment because it is impossible to find a suitable donor. This is one reason why researchers are enthusiastic about umbilical blood stem cells.
Collected from the discarded umbilical cords of newborn children, umbilical blood stem cells are more adaptable, and so there is less need for a precise match between the cord cells and the DNA of the donor.
Such cells have been used to treat a range of conditions, including cerebral palsy. Both Canada and Britain have developed national cord blood banks, which allow new mothers to donate cord blood after giving birth.
Faster, smarter drug testing
Another breakthrough lies in the manipulation of adult stem cells. In 2007, scientists discovered it was possible to turn adult stem cells into induced pluripotent stem cells (iPS cells), which are able to produce a range of different types of cell.
Indeed, because iPS cells are very similar to embryonic stem cells, they could end up making research on the latter redundant. iPS cells have been used to grow a wide range of tissue types, including brain tissue.
This ability to grow human tissue in the lab has huge implications for medical research and development. Currently, scientists studying the effects of treatments have a limited range of options, all with serious drawbacks. They can use computer models, animal testing, or run clinical trials on humans.
Computer modelling relies on the assumptions put into the model, and is generally seen by regulators as insufficient as evidence on its own. Human trials are expensive, and have obvious safety issues.
So, a lot of early-stage trials involve animals. Animal testing is a useful tool that has saved lives and money, but it is also imperfect. Human and animal physiology is similar enough to be scientifically useful, but there are some key differences in how different species react to certain compounds.
In many cases, drugs that work well for animals fail, or are even toxic, for humans. It also works the other way, so that some drugs that may have been effective in humans are perhaps unnecessarily rejected because they failed to work in animal tests.
So, the hope is that these lab-created human tissues could be used for research and even early-stage treatment trials. As the structures built this way grow ever more sophisticated, their usefulness will increase.
A team at the University of Queensland's Institute for Molecular Bioscience recently grew a fully functioning miniature kidney from modified adult skin stem cells.
Growing new organs
Researchers are even starting to create whole organs for transplantation. Wake Forest School of Medicine in North Carolina has been growing bladders and transplanting them into patients with spina bifida since 2006.
Follow up research suggests that they improved bladder function in all patients. At the moment they are licensing the treatment to biotech company Tengion, which is trying to develop it commercially.
Regular adult stem cells are also being used to assist in transplants. The stem cells are seeded onto a scaffold' tissue from a donor (or synthetic tissue) that serves as a platform for the cells to grow on.
Even if they only function as a lining to the donor organ, this helps the body to accept the transplant, reducing the need for expensive medication.
In 2008, the world's first engineered trachea transplant was carried out on a Colombian woman, who is still alive today you can read more on this story and more recent developments below.
The firm behind the bioreactor' responsible for turning stem cells into this lining, Harvard Apparatus Regenerative Technology (HART) is hoping this process can be applied to other, more complicated organs.
In recent pre-clinical trials, similar methods were used to carry out a heart transplant on arat. Work on creating heart valves that can be used in humans is being carried out in conjunction with the world-famous Mayo Clinic, while other potential organ candidates include the lungs and general intestine tract.
Further into the future, the hope is that entire vital organs (such as hearts or livers) could be grown. Already researchers at Yokohama City University have transplanted mini-livers' into mice, which were accepted by their hosts and worked without any problems.
While experts caution that it will be at least a decade before lab-grown human vital organs are usable in this way, parts of organs could be grafted onto diseased or damaged organs much sooner.
Four inspiring success stories
In 2008, Claudia Castillo, a Colombian woman whose trachea (part of her windpipe) had collapsed following a bout of tuberculosis, received a transplant spare part' created from stem cells harvested from her own bone marrow.
Trachea tissue taken from a dead donor was used as a scaffold, onto which Castillo's own stem cells were seeded. Researchers at the University of Bristol then turned the stem cells into the cartilage cells that coat windpipes, and Castillo received the transplant, which her body accepted.
In 2009,part of a jawbone one of the most complex bones in the body was grown in a similar way by researchers at New York's Columbia University. And in 2011, in Sweden, the first successful transplant using a scaffold built from a completely synthetic material was carried out, removing the need for any third-party donor.
In early 2009, medics at the St Josef-Hospital, part of the Ruhr-University of Bochum, treated a two-year-old German boy with infantile cerebral palsy using stem cells from his own umbilical cord blood.
The boy had suffered a heart attack in late 2008, which had left him paralysed and in a vegetative state, with his chances of survival put at 6%.
However, following the cord blood therapy, the boy recovered "relatively quickly", and by mid-2012 he was able to eat, walk with assistance and form sentences.
In 2013, a team from the University of Pittsburgh, Pennsylvania, building on similar work done by researchers in Japan, constructed human heart tissue from iPS cells (see main story) taken from adult skin.
While the tissue was grown using a mouse heart as a scaffold, the mouse cells were removed so that the resulting tissue was entirely human. At present, while they have only grown part of a human heart, it is beating healthily.
The six stocks to buy now
As with most cutting-edge areas of biotechnology, if you want to get full exposure to the adult stem cell sector, you'll have to be prepared to invest in small, risky companies.Most firms are still far away from reaching profitability, with their value lying in the promise of future revenues.
If you'd rather stick with bigger players, a few larger companies are starting to invest in the sector. Foremost is drug company Teva Pharmaceutical Industries (NYSE: TEVA).
While Teva is well known for its generic (non-branded) drug business, it has been partnering with biotech firms and is staging a final phase trial of Revascor, an adult stem cell treatment for congestive heart failure, involving 1,700 patients. Teva trades at 8.8 times its 2015 earnings forecasts.
Tom Bulford of the Red Hot Biotech Alert newsletter likes the look of Harvard Apparatus Regenerative Technology (Nasdaq: HART). HART is a recent spin-off from Harvard Biosciences.
It makes a bioreactor' that is used to turn stem cells into the linings for replacement organs, and the scaffolds such organs are created on. This year it expects to continue with trials in the US, Europe and Russia and to get several new patents approved.
It also hopes to win orphan' treatment designation (which makes it easier to get approval for use from the US regulators).
The Japanese government is making a big effort to encourage adult stem cell research, with the hope of turning it into a major strategic industry. Last year, Japan's parliament passed laws streamlining the approval process and dramatically increased government funding.
One firm that will benefit from this is Japan Tissue Engineering Company (Tokyo: 7774), which focuses on producing human tissues from adult stem cells. It has already won approval for the production of human cartilage and skin. It's not yet profitable, but a parternship with Fujifilm (its largest shareholder) should ensure its financial security in the meantime.
StemCells Inc (Nasdaq: STEM) is one of the oldest companies in the sector, having been listed since 1992. It focuses on using stem cells to repair damage to the central nervous system (such as the brain and the spinal cord).
Data from its latest international trials, which have been expanded to North America, suggest that the company's techniques are not only safe, but have also produced an improvement in the condition of patients who had been paralysed. Even more positively, this improvement seems to be permanent rather than temporary.
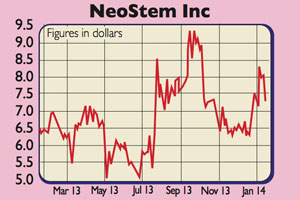
When we tipped NeoStem (Nasdaq: NBS) last March (issue 630), it was trading at $6. It's gone up by nearly 20% since, and continues to make progress with its various trials. These involve using VSELs (a type of adult stem cell) on conditions including heart disease, diabetes and gum disease.
NeoStem has also taken out several new patents and announced its intention to launch trials for other problems, such as heart failure and brain injury. It has plenty of cash to tide it over while these trials continue.
Brainstorm Cell Therapeutics (OTC: BCLI) is based around just one product, NurOwn. This uses bone-marrow-derived adult stem cells to treat degenerative diseases, most notably motor neurone disease.
Initial results have been positive, with early trials showing that the process can slow down the disease's progression. In one case a patient who had been unable to speak was able to deliver a speech in front of an audience.
Brainstorm has won orphan drug' approval from the US regulator, which should speed up the approval process. The shares are sold over the counter', so you'll need a broker who can trade these stocks.
Get the latest financial news, insights and expert analysis from our award-winning MoneyWeek team, to help you understand what really matters when it comes to your finances.

-
 MoneyWeek Talks: The funds to choose in 2026
MoneyWeek Talks: The funds to choose in 2026Podcast Fidelity's Tom Stevenson reveals his top three funds for 2026 for your ISA or self-invested personal pension
-
 Three companies with deep economic moats to buy now
Three companies with deep economic moats to buy nowOpinion An economic moat can underpin a company's future returns. Here, Imran Sattar, portfolio manager at Edinburgh Investment Trust, selects three stocks to buy now
-
 Invest in space: the final frontier for investors
Invest in space: the final frontier for investorsCover Story Matthew Partridge takes a look at how to invest in space, and explores the top stocks to buy to build exposure to this rapidly expanding sector.
-
 Invest in Brazil as the country gets set for growth
Invest in Brazil as the country gets set for growthCover Story It’s time to invest in Brazil as the economic powerhouse looks set to profit from the two key trends of the next 20 years: the global energy transition and population growth, says James McKeigue.
-
 5 of the world’s best stocks
5 of the world’s best stocksCover Story Here are five of the world’s best stocks according to Rupert Hargreaves. He believes all of these businesses have unique advantages that will help them grow.
-
 The best British tech stocks from a thriving sector
The best British tech stocks from a thriving sectorCover Story Move over, Silicon Valley. Over the past two decades the UK has become one of the main global hubs for tech start-ups. Matthew Partridge explains why, and highlights the most promising investments.
-
 Could gold be the basis for a new global currency?
Could gold be the basis for a new global currency?Cover Story Gold has always been the most reliable form of money. Now collaboration between China and Russia could lead to a new gold-backed means of exchange – giving prices a big boost, says Dominic Frisby
-
 How to invest in videogames – a Great British success story
How to invest in videogames – a Great British success storyCover Story The pandemic gave the videogame sector a big boost, and that strong growth will endure. Bruce Packard provides an overview of the global outlook and assesses the four key UK-listed gaming firms.
-
 How to invest in smart factories as the “fourth industrial revolution” arrives
How to invest in smart factories as the “fourth industrial revolution” arrivesCover Story Exciting new technologies and trends are coming together to change the face of manufacturing. Matthew Partridge looks at the companies that will drive the fourth industrial revolution.
-
 Why now is a good time to buy diamond miners
Why now is a good time to buy diamond minersCover Story Demand for the gems is set to outstrip supply, making it a good time to buy miners, says David J. Stevenson.