Stock Markets
The latest news, updates and opinions on Stock Markets from the expert team here at MoneyWeek
Explore Stock Markets
-
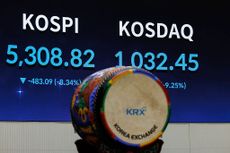
Stock market circuit breaker: Why did Korean shares pause trading?
The fallout from the conflict in the Middle East hit the Korean stock market on 4 March, with shares forced to temporarily stop trading. What is a stock market circuit breaker, and why did the KOSPI trigger one?
By Dan McEvoy Published
-

Why do experts think emerging markets will outperform?
Emerging markets were one of the top-performing themes of 2025, but they could have further to run as global investors diversify
By Dan McEvoy Published
-

Investing in Taiwan: profit from the rise of Asia’s Silicon Valley
Taiwan has become a technology manufacturing powerhouse. Smart investors should buy in now, says Matthew Partridge
By Dr Matthew Partridge Published
-

How to invest as the shine wears off consumer brands
Consumer brands no longer impress with their labels. Customers just want what works at a bargain price. That’s a problem for the industry giants, says Jamie Ward
By Jamie Ward Published
-

A niche way to diversify your exposure to the AI boom
The AI boom is still dominating markets, but specialist strategies can help diversify your risks
By Cris Sholto Heaton Published
-

New PM Sanae Takaichi has a mandate and a plan to boost Japan's economy
Opinion Markets applauded new prime minister Sanae Takaichi’s victory – and Japan's economy and stockmarket have further to climb, says Merryn Somerset Webb
By Merryn Somerset Webb Published
Opinion -

Can US small caps survive the software selloff?
US stocks have made their worst start to a year since 1995 relative to a global benchmark. But experts think some sectors of the market are still worth buying.
By Dan McEvoy Published
-

Could Chinese investments race ahead in the Year of the Horse?
As the Year of the Horse begins, we highlight the trends and sectors that could make great Chinese investments for the coming Lunar year
By Dan McEvoy Published
-

Japanese stocks rise on Takaichi’s snap election landslide
Japan’s new prime minister Sanae Takaichi has won a landslide victory in a snap election, prompting optimism that her pro-growth agenda will benefit Japanese stocks
By Dan McEvoy Published