The unscientific criticism of AstraZeneca could do lasting damage
Several European countries have suspended their use of AstraZeneca's Covid vaccine – for no good scientific reason. Matthew Partridge reports.

Get the latest financial news, insights and expert analysis from our award-winning MoneyWeek team, to help you understand what really matters when it comes to your finances.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?
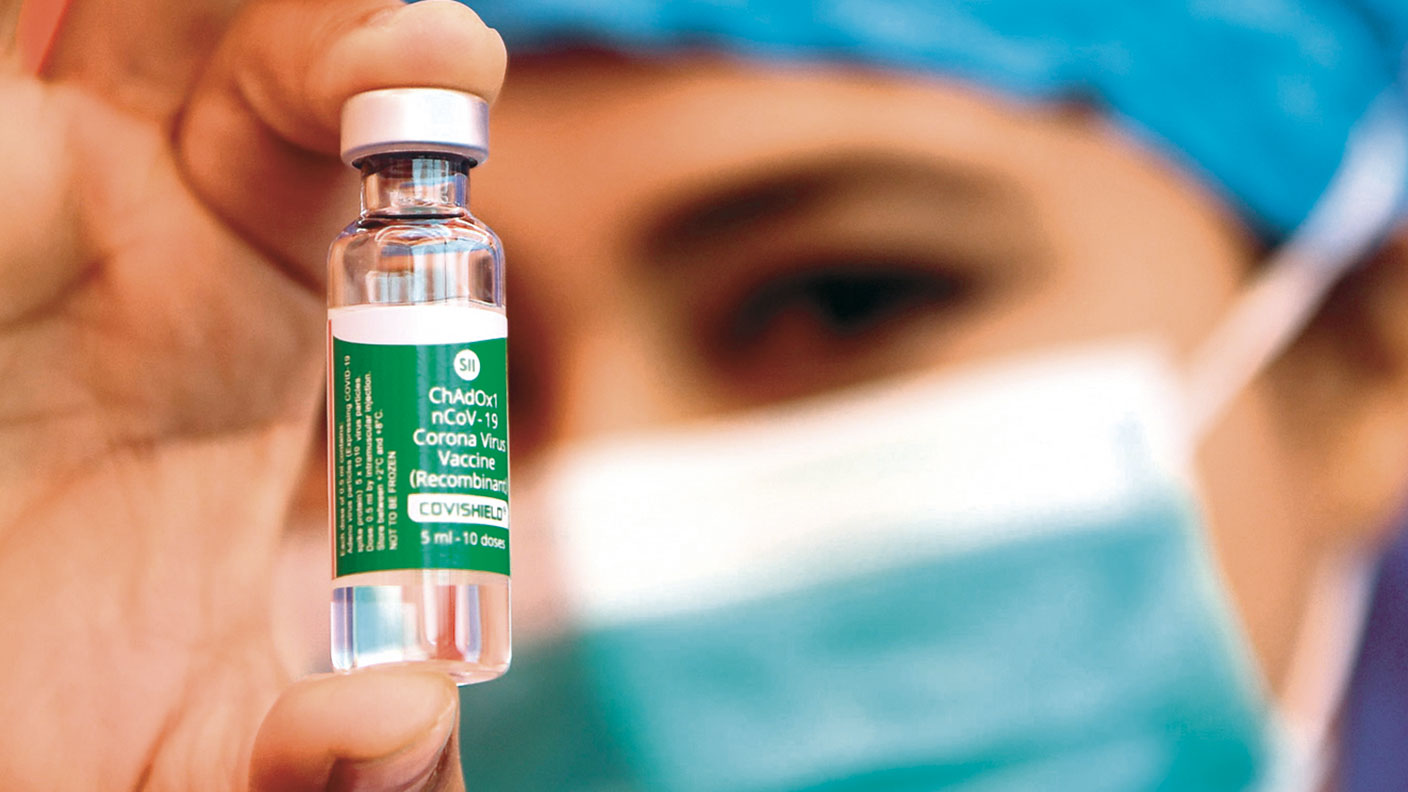
The AstraZeneca vaccine has suffered a setback in the past few days as France, Germany and Italy have joined a “growing list” of European countries, now more than a dozen in total, to halt its use “temporarily”, says The Economist. A few countries in the rest of the world, including Indonesia, have followed suit. This “wave of suspensions” has been set in motion by data from a Norwegian medical regulator reporting four cases of blood clotting in adults given the vaccine. Similar scattered reports of blood clots “have come from Denmark, Italy and Austria”.
Those countries suspending the vaccine are behaving irrationally, says David Spiegelhalter in The Guardian. While suspected reactions “should be investigated”, moving too fast may lead to regulators “drawing causal links between events where none may exist”.
Given that one in 5,000 people end up having a blood clot each year, it is “not at all surprising” that there have been 30 reports of blood clots so far from the five million people who have had AstraZeneca’s vaccine, especially since most vaccinations have occurred among the elderly and infirm. This hardly means the vaccine caused the clots. If anything, all the data so far has demonstrated how “extraordinarily safe” both the AstraZeneca and the Pfizer vaccines are.
Try 6 free issues of MoneyWeek today
Get unparalleled financial insight, analysis and expert opinion you can profit from.

Sign up to Money Morning
Don't miss the latest investment and personal finances news, market analysis, plus money-saving tips with our free twice-daily newsletter
Don't miss the latest investment and personal finances news, market analysis, plus money-saving tips with our free twice-daily newsletter
Lasting reputational damage
Experts agree that the decisions to suspend the vaccine look wrong, especially since the European Medicines Agency (EMA), the EU’s main regulator, continues to defend it, says Paul Cullen in the Irish Times. However, even if these countries eventually change their minds, the damage to the reputation of the AstraZeneca vaccine will be “massive and possibly lasting”. Despite its “impressively high effectiveness in real-world trials”, disparaging comments by many European politicians, as well as a perception that it is less effective in older patients, mean that it is facing a high degree of “consumer resistance”.
Concern over blood clots and lower effectiveness aren’t the only problem that AstraZeneca is up against, say Suzi Ring and Michelle Fay Cortez on Bloomberg. There is also the ongoing controversy over “manufacturing issues”, which means that the company will “only be able to deliver about 100 million doses to the EU in the first half of the year”, about one-third of the number originally planned.
AstraZeneca’s loss could be other companies’ gain, says Aimee Donnellan on Breakingviews. The EU has set itself a target of vaccinating 70% of its population, around 350 million people, by the summer. But without AstraZeneca’s vaccines, it will only have enough for 250 million people. So if Europe is to avoid having to impose more lockdowns in the winter, when infections start to pick up, Brussels will need to “speedily approve and distribute” other candidates, such as Johnson & Johnson’s jab, which got the nod from the EMA last week.
Get the latest financial news, insights and expert analysis from our award-winning MoneyWeek team, to help you understand what really matters when it comes to your finances.

-
 Has the market misjudged Relx?
Has the market misjudged Relx?Relx shares fell on fears that AI was about to eat its lunch, but the firm remains well placed to thrive
-
 Dario Amodei: The AI boss in a showdown with Trump
Dario Amodei: The AI boss in a showdown with TrumpAnthropic’s CEO Dario Amodei was on an extraordinary upward trajectory when he found himself on the wrong side of the American president. He is about to be severely tested.
-
 Has the market misjudged Relx?
Has the market misjudged Relx?Relx shares fell on fears that AI was about to eat its lunch, but the firm remains well placed to thrive
-
 8 of the best properties for sale with minstrels’ galleries
8 of the best properties for sale with minstrels’ galleriesThe best properties for sale with minstrels’ galleries – from a 15th-century house in Kent, to a four-storey house in Hampstead, comprising part of a converted, Grade II-listed former library
-
 The rare books which are selling for thousands
The rare books which are selling for thousandsRare books have been given a boost by the film Wuthering Heights. So how much are they really selling for?
-
 How to invest as the shine wears off consumer brands
How to invest as the shine wears off consumer brandsConsumer brands no longer impress with their labels. Customers just want what works at a bargain price. That’s a problem for the industry giants, says Jamie Ward
-
 A niche way to diversify your exposure to the AI boom
A niche way to diversify your exposure to the AI boomThe AI boom is still dominating markets, but specialist strategies can help diversify your risks
-
 New PM Sanae Takaichi has a mandate and a plan to boost Japan's economy
New PM Sanae Takaichi has a mandate and a plan to boost Japan's economyOpinion Markets applauded new prime minister Sanae Takaichi’s victory – and Japan's economy and stockmarket have further to climb, says Merryn Somerset Webb
-
 Early signs of the AI apocalypse?
Early signs of the AI apocalypse?Uncertainty is rife as investors question what the impact of AI will be.
-
 8 of the best properties for sale with beautiful kitchens
8 of the best properties for sale with beautiful kitchensThe best properties for sale with beautiful kitchens – from a Modernist house moments from the River Thames in Chiswick, to a 19th-century Italian house in Florence